Eye drop recall
Web The Food and Drug Administration posted separate recall notices for certain eyedrops distributed by Pharmedica and Apotex after the companies said they are voluntarily pulling several lots of their products from the market. The drops have not been linked to illness the company said though it.

Eye Drops Recalled Over Potential Life Threatening Infections
UC Davis Health experts share what you need to know.

. Web An alarming outbreak of extensively drug-resistant bacteria linked to eye drops has now sickened 68 people across 16 states according to the latest update from the Centers for Disease Control. Web The Food and Drug Administration FDA has recently recalled three brands of eye drops including one that has been linked to serious infections vision loss and a death. Web 9 hours agoPharmedica USA in March recalled Purely Soothing 15 MSM Drops out of concern that the product is not sterile.
Both companies said the recalls were conducted in consultation with the FDA. Web 1 day agoHealth Manufacturer recalls eyedrops after possible link to bacterial infections Per the CDCs latest update 68 patients across 16 states have been infected with Pseudomonas aeruginosa. Consumers are advised to stop using the following brands and return them to the place of purchase.
A majority of those affected reported using preservative-free EzriCare Artificial Tears before they became. Web WASHINGTON US. The two lots were pulled because of problems that could result in blindness the company said.
Web Two types of artificial tears eye drops have been voluntarily recalled following 55 reports of adverse use effects including eye infections vision loss and even a bloodstream infection that led. Web Bacteria in recalled eye drops linked to cases of vision loss surgical removal of eyeballs Global Pharma Healthcare recalled its Artificial Tears Lubricant Eye Drops that were. Health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and serious injury.
Web On Thursday the maker of the eyedrops recalled them because of possible contamination. Web Pharmedica is recalling its Purely Soothing 15 MSM Drops meant to treat eye irritation.

Eyedrops Recalled Due To Contamination Risks That Could Lead To Serious Injury Npr

Certain Pharmasave Advanced Relief Eye Drops And Compliments Advanced Relief Eye Drops Recalled
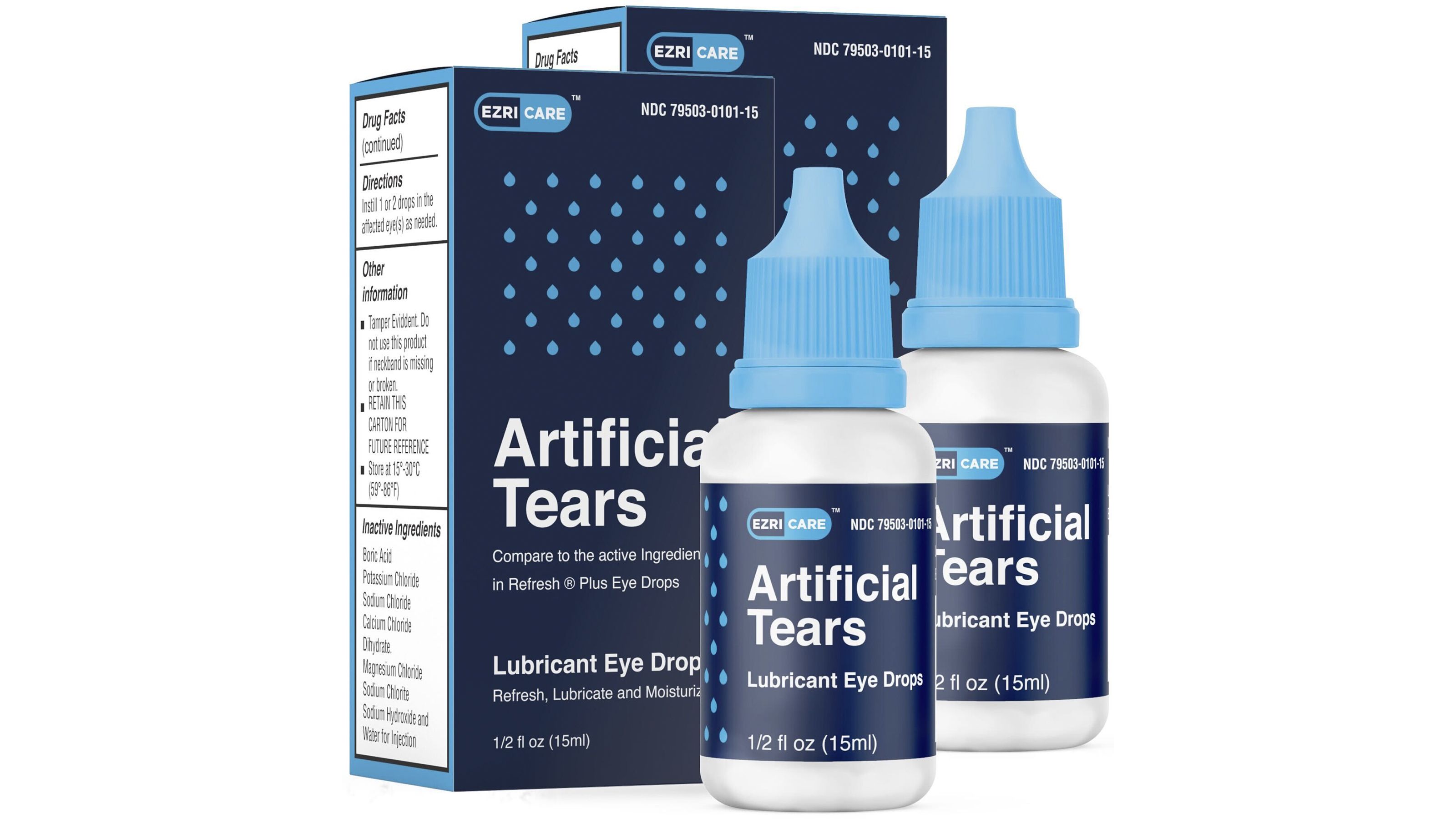
Bacteria In Recalled Eye Drops Linked To Cases Of Vision Loss Surgical Removal Of Eyeballs Cnn

More Otc Eye Drops Recalled Amid Bacterial Contamination Fears

Wppiufxxa8rv M

O N2vpc3liz1vm

Eye Drops Recalled Over Non Sterility Fda Fox Business

H7p6185xwbxylm

Fda Expands Recall Of Eye Drops Linked To Bacterial Contamination Top Class Actions

Eye Product Recall Fda Expands Warning Over Contaminated Eye Drops To Include Delsam Pharma S Artificial Eye Ointment Amid Bacterial Outbreak Cbs News

Chennai Firm Recalls Eye Drops After Us Flags Vision Loss Death
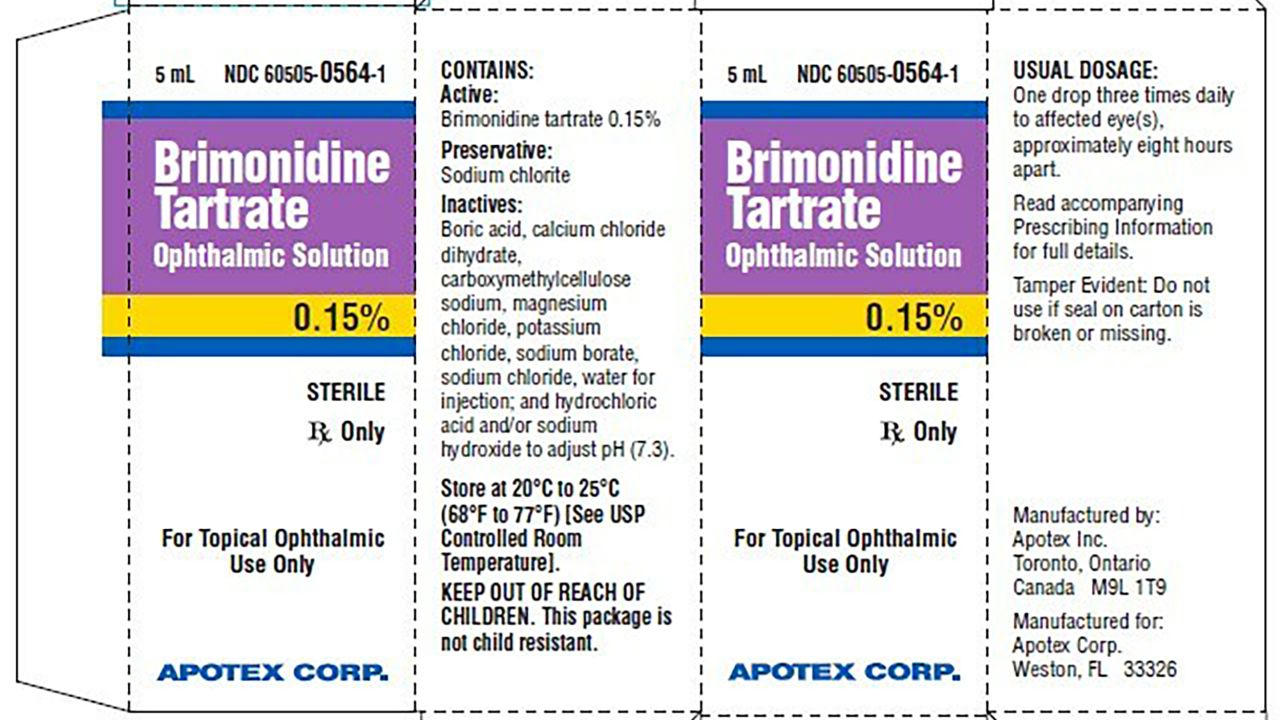
O N2vpc3liz1vm

O N2vpc3liz1vm

Altaire Recalls Eye Drops Ointments Sold At Walgreens Healthcare Packaging

Eye Drops Recalled By Two Companies Over Safety Concerns Wsj

Third Eye Drop Brand Recalled One Linked To Serious Infection Vision Loss And A Death Myparistexas
Eyedrop Recall Fda Adds 2 More Brands To List Due To Infection Risk Bloomberg